
Azo Initiators | Polymerization Solutions
Azo Initiators | Polymerization Solutions
Azo Initiators
High-Purity Azo Initiators for Precision Polymerization
The industry standard source for AIBN, V-50, and custom Azo compounds. V
erified kinetic data and >99.5% purity for controlled radical polymerization.
What is an Azo Initiator?
An Azo Initiator is a chemical compound containing the functional group R-N=N-R'. Upon heating or irradiation, it decomposes to release nitrogen gas (N2) and two carbon-centered free radicals. These radicals initiate the polymerization of monomers such as styrene, acrylates, and vinyl chloride. Azo initiators are preferred over peroxides for their predictable first-order decomposition kinetics and lack of induced decomposition.
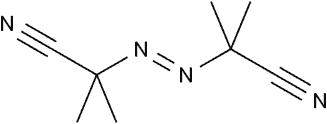
2,2'-Azobis(2-methylpropionitrile)
CAS: 78-67-1
Short Name:AIBN, V-60
Purpose:One of the most commonly used oil-soluble initiators, with a decomposition temperature of about 65℃, is widely applied in the synthesis of general-purpose plastics and rubbers.
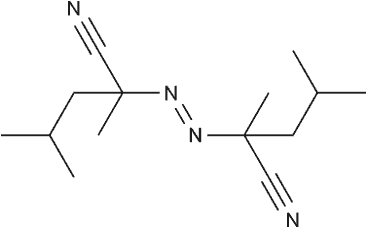
2,2'-Azobis(2,4dimethylvaleronitrile)
CAS:4419-11-8
Short Name:V-65/ABVN
Purpose:It exhibits higher activity and has a lower decomposition temperature (50-65℃), making it suitable for polymerization reactions that require low-temperature initiation
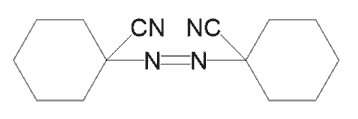
1,1-Azodi(hexahydrobenzonitrile)
CAS:2094-98-6
Short Name:V-40/ACCN
Purpose:Primarily used as a polymer initiator to initiate radical reactions
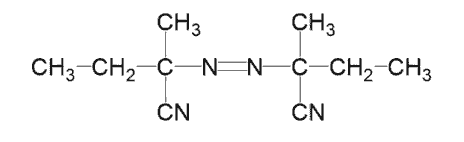
2,2'-Azodi(2-methylbutyronitrile)
CAS:13472-08-7
Short Name:V-59/AMBN
Purpose:Used as a polymerization initiator for vinyl compounds
Azo Initiator Selection Guide
Use the table below to select the appropriate initiator based on solubility and decomposition temperature.
AIBN
Chemical Name:2,2'-Azobis(2-methylpropionitrile)
CAS Number:78-67-1
10h Half-Life Temp (T10h):65°C
Solubility:Toluene, Methanol, Alcohol
AMBN
Chemical Name:2,2'-Azobis(2-methylbutyronitrile)
CAS Number:13472-08-7
10h Half-Life Temp (T10h):67°C
Solubility:Aromatic Hydrocarbons, Acetone
V-50
Chemical Name:2,2'-Azobis(2-methylpropionamidine) dihydrochloride
CAS Number:2997-92-4
10h Half-Life Temp (T10h):56°C
Solubility:Water (Soluble)
V-601
Chemical Name:Dimethyl 2,2'-azobis(2-methylpropionate)
CAS Number:2589-57-5
10h Half-Life Temp (T10h):66°C
Solubility:Organic Solvents
ABCN
Chemical Name:1,1'-Azobis(cyclohexanecarbonitrile)
CAS Number:2094-98-6
10h Half-Life Temp (T10h):88°C
Solubility:Toluene, Organic Solvents
Why Choose Azo Compounds over Peroxides?
Controlled Kinetics
Azo decomposition follows strict first-order kinetics (rate = kd[I]), allowing for precise calculation of polymerization rates and molecular weight control.
Linear Polymers
Unlike organic peroxides, Azo initiators do not engage in hydrogen abstraction, resulting in linear polymers with minimal branching and better mechanical properties.
Safety & Stability
While still requiring cold storage, Azo compounds generally have higher self-accelerating decomposition temperatures (SADT) than comparable peroxides, offering safer handling.
The Problem: Inconsistent Polymerization Impacts Yield
Decomposition temperature and control
A mismatch in decomposition temperature or too rapid decomposition can lead to intense reactions, concentrated heat release, broadened molecular weight distribution, and unstable yield.
Solubility and dispersion
Poor solubility or dispersibility of the initiator in the reaction system can lead to locally high concentrations, uneven initiation, and significant differences in the rate of polymerization reactions in different regions, affecting the uniformity and overall yield of the product.
Initiator efficiency
The initiator efficiency f-value (representing the proportion of free radicals used to initiate polymerization) is typically less than 1. Factors such as cage effect and induced decomposition can lead to a reduction in the actual number of free radicals used to initiate polymerization, necessitating the use of more initiators or extending the reaction time, which may introduce the risk of side reactions.
Impurity sensitivity
Although azo initiators have relatively low sensitivity to impurities, the presence of impurities such as metal ions (e.g., Cu²⁺) in the polymerization system may still inhibit polymerization or affect the decomposition of the initiator, leading to incomplete reaction and decreased yield.
Storage and stability
Improper storage conditions (such as excessive temperature and exposure to light) for azo-based initiators may lead to their slow decomposition or loss of effectiveness. The use of such initiators may result in insufficient initiation efficiency due to a decrease in the actual active ingredient content, leading to slow or non-occurrence of reactions and low yields.
Our Solution:
Enhance the stability and predictability of polymerization yield
Precisely match the initiator with the reaction conditions:
Select azo-based initiator varieties with a half-life temperature (T1/2) that matches the target polymerization temperature. For heat-sensitive polymerization systems, consider using redox initiation systems to initiate polymerization at lower temperatures.
Ensure uniform dispersion of the initiator:
Select an initiator with solubility matching the polymerization system (aqueous solution, organic phase, etc.) (water-soluble such as AIBA, oil-soluble such as AIBN). If necessary, pre-dissolution, efficient stirring, or emulsification methods can be used to promote uniform distribution of the initiator in the system.
Optimize the purity and conditions of the reaction system:
Strictly control the purity of monomers and solvents to reduce the introduction of impurities, especially metal ions.Strengthen heat and material transfer during the reaction process by optimizing stirring, heat exchange, and other methods.
Regulate the storage and use of initiators:
Strictly follow the product instructions and store azo-based initiators under recommended conditions (such as low temperature and away from light). Confirm their good condition before use, and avoid using deteriorated or uncertainly active initiators

Key Features and Technical Advantages
Azo-based initiators are a crucial class of radical polymerization initiators, characterized primarily by their ability to generate free radicals through thermal decomposition, accompanied by the release of nitrogen gas.
Decomposition mechanism
During thermal decomposition, homolysis of the N-N bond occurs, generating two carbon radicals and nitrogen gas, following first-order reaction kinetics.
The decomposition rate is controllable and highly predictable; the decomposition only produces a single type of free radical, with fewer side reactions, resulting in a more regular polymer structure.
The common induced decomposition and solvent effects of peroxides are avoided, resulting in a more concise reaction pathway.
Reaction product
The decomposition products are carbon radicals and nitrogen gas.
The generation of nitrogen can simultaneously serve as a foaming agent in the production of foam plastics; carbon-centered radicals are less prone to hydrogen abstraction reactions, which is conducive to the formation of linear polymers. Peroxides may generate oxygen or oxygen-containing radicals, sometimes leading to side reactions such as branching or crosslinking.
Storage and operational safety
Stable at room temperature, it can usually be stored for over 1 year; it is insensitive to friction and impact.
The safety of storage and transportation has been significantly enhanced; some new varieties (such as AIBME) have reduced toxicity through structural improvements. Peroxides are generally more sensitive to heat and friction, and require more stringent storage conditions.
Application flexibility
The decomposition temperature range is wide (typically 40-100℃), and can be finely adjusted by altering the R group (such as introducing polar groups like —CN, —COOR, etc.). The most suitable initiator can be selected based on the polymerization system (solvent type, polymerization temperature) and process requirements (solution polymerization, lotion polymerization)
Applications in Polymer Manufacturing
PVC Production
As an oil-soluble initiator, it provides the initial free radical in radical polymerization reactions, initiating the chain growth reaction of vinyl chloride monomer.Azobisisobutyronitrile (AIBN) and azobis(2-amidinopropionitrile) (ABVN) are commonly used varieties in suspension polymerization and microsuspension polymerization.
The decomposition process is smooth, mainly producing nitrogen with few side reactions, which helps to generate PVC resin with a relatively uniform molecular weight distribution. It is mainly used in suspension polymerization (the most important production process of PVC) and microsuspension polymerization (for producing PVC paste resin)
Polyethylene (PE) Synthesis
Azo initiators are primarily used as initiators for radical polymerization in the synthesis of polyethylene.
Azobisisobutyronitrile (AIBN) is one of the commonly used varieties.
The decomposition reaction is smooth, basically producing only one type of free radical, with few side reactions, which are easy to control, making it suitable for research on polymer synthesis.
Azo-based initiators such as AIBN are more commonly used in some low-temperature solution polymerizations or specific research scenarios due to their decomposition temperature characteristics.
Acrylic Resins
As an oil-soluble initiator, it decomposes under heating conditions to generate free radicals, which initiate the radical polymerization of monomers such as acrylate and methacrylate.
Azobisisobutyronitrile (AIBN) is the most representative variety, and azobisisobutyronitrile (ABVN) is also used.
The decomposition method is simple, with fewer side reactions, resulting in a narrower molecular weight distribution and a more regular structure of the obtained polymer.
The decomposition products do not contain oxygen, which makes them less prone to cause the polymer to oxidize and turn yellow, helping the resin maintain a lighter color and better outdoor durability. Storage and handling are relatively safer. It is mainly used in solution polymerization and bulk polymerization processes and is a common choice for synthesizing thermoplastic acrylic resins
Trust and Credibility
At Azo Initiators, we specialize in providing high-purity azo initiators designed to enhance polymerization processes across various industries. Our commitment to quality ensures that each product meets stringent standards for performance and safety.
Download Technical Data Sheets
Check the details of our AZOX Azo initiators series products
Frequently Asked Questions (FAQ)
Your Questions, We Value.
What are azo initiators?
What are the benefits of using V-Series azo initiators?
Are your products cyanide-free?
Contact Us
We'd love to hear from you. Your inquiries are important to us.
Room 1201-1212,Jinda Mansion, Liuquan Road, Zhangdian District, Zibo, Shandong, China.+86-15166012761Select...

About Us
Products
Contact Us




